Membrane fouling in MBRs

Membrane fouling
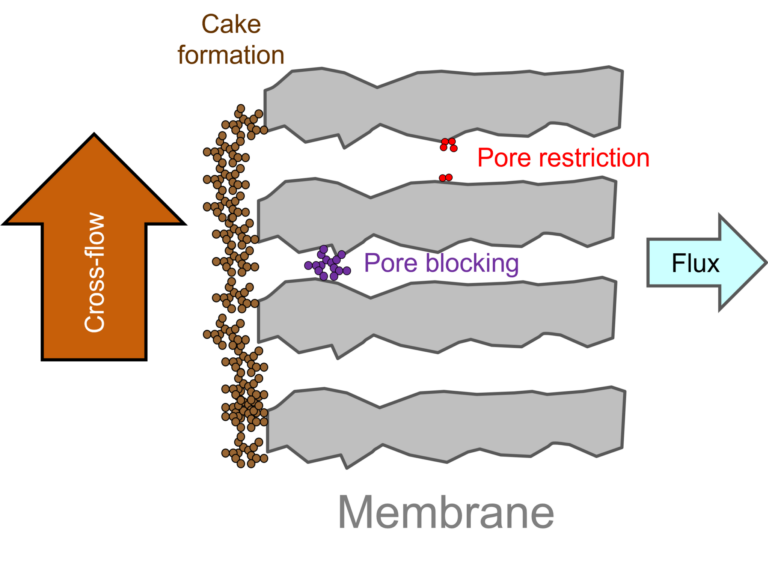
'Membrane fouling' is the general term given to the impeding of flow through the membrane due to:
- the plugging or partial plugging of the membrane pores by fine particulate or colloidal material, and/or
- the build up of a layer of relatively impermeable material, referred to as the 'cake' or 'fouling' layer, at the membrane surface.
Fouling in MBRs is caused by dissolved and colloidal polymeric species in the mixed liquor, generally associated with the microbial species. These foulants are variously referred to as extracellular polymeric substances (EPS), soluble microbial product (SMP) and biopolymer clusters, along with a number of other terms. A large amount of academic research has been devoted to the physical and chemical characterisation of these foulants, particularly by fractionating them into different chemical groups (such as proteins and polysaccharides).
Organic foulants generally impede the flow of water through the hydrophobicity of the foulant material. A key facet of successful MBR operation is the maintainence of hydrophilic conditions at the membrane−solution interface. This can be achieved by initial 'wetting out' of the membrane, if it is a hydrophobic polymer, or by selection of a hydrophilic membrane material such as ceramics.
Fouling also takes place through the action of certain sparingly-soluble inorganic materials, such as ferric oxide and calcium carbonate, which can precipitate onto the membrane surface. These foulants are referred to as 'scalants', and are less prevalent in MBRs than in dense membrane processes such as reverse osmosis.
The impeding of flow through the membrane pores due to pore restriction or plugging ((i) above) is less common in MBRs than in conventional membrane filtration. This is because the membrane is challenged with extremely high suspended solids concentrations, which form a protective cake layer on the membrane surface.
Cake layer formation ((ii) above) is the most common manifestation of fouling. The cake layer is generally at least ten times less permeable than the membrane on which it sits. The extent of fouling is controlled by:
- applying shear to the membrane, either by:
- the action of the retentate liquid flowing parallel to the membrane surface (so-called 'crossflow' filtration), or
- the action of air bubbles travelling up through the membrane channels
- periodically halting the flow of permeate through the membrane to allow the air bubbles to scour the membrane (a mode of physical cleaning known as ‘relaxation’)
- periodically halting the flow of permeate and reversing the flow back through the membrane (known as ‘backflushing’ or ‘backwashing’)
- the use of chemicals to clean the membrane.
There have been various novel methods proposed for controlling fouling, mostly arising from the plethora of academic research into fouling phenomena. These can be roughly divided into:
- membrane surface modification to increase hydrophilicity
- biological fouling prevention methods, such as quorum quenching
- biochemical cleaning using enzymes
- enhanced physical cleaning, such as through electrochemical or ultrasonic assistance, and
- chemical dosing to limit the propensity of the foulants to form an impermeable cake layer.
Of the above, examples of innately hydrophilic membrane materials which have been successfully implemented include ceramic membranes such as alumina and silicon carbide (SiC). There are few commercial examples of hydrophilic surface modification of innately hydrophobic polymeric membrane materials.
Biological, biochemical and enhanced physical cleaning methods appear not to have been extensively applied at full scale. There are some reported instances of the addition of media, such as powdered activated carbon or plastic media, which appear to have a small impact on flux. Such media are intended primarily to enhance the biodegradation of biorefractory organic species, but may have an additional secondary benefit of flux enhancement.
Of the methods listed, only chemical dosing appears to have been commercialised. Organic polymers have been dosed into the mixed liquor specifically to suppress membrane fouling, with some apparent success. A few chemical suppliers offer bespoke chemicals for this duty.
Membrane surface fouling is to be distinguished from membrane channel clogging, where the mixed liquor suspended solids agglomerate between the membrane channels and partially or completely block the channel.








