Ammonia recovery from sludge liquors (*WNWNW #5)
Simon Judd has over 35 years’ post-doctorate experience in all aspects of water and wastewater treatment technology, both in academic and industrial R&D. He has (co-)authored six book titles and over 200 peer-reviewed publications in water and wastewater treatment.
Waste Not Want Not Wednesdays #5: the benefit of recovering ammonia from sludge processing streams*
The removal of ammonia and Kjeldahl nitrogen from sewage is pretty well-established. It dates back as far as the early 1910s, to the initial studies conducted in the US (Clark and Gage, 1912, 1913) very rapidly followed by the seminal work of Ardern and Lockett (1914a,b) in the UK. By 2014, the first activated sludge plant was implemented at Davyhulme in the UK, and the rest is history.
It’s hard not to be admire the work of these early pioneers – even though it took a while longer to work out how the process actually worked. The simple fact is that, more than 110 years later, we’re still using technologies based on the same process to simultaneously biochemically mineralise carbon and ammonia. The process is robust, well understood, and very effective. Indeed, achieving the same mineralisation efficiency using a physicochemical process would demand something like supercritical water oxidation, which would certainly keep the HAZOP officers busy. What’s not to like?
Well, the simple answer is: energy consumption. The classical process involves oxidising ammonia to nitrate (nitrification) – a change in oxidation number of +8 – followed by reduction of the nitrate to nitrogen gas (denitrification). The oxidation step demands a fair amount of air, and aeration = energy. So, the sensible thing to do is simply to physically remove the ammonia. However, this is not without challenges.
On the face of it, recovery of ammonia from sewage is subject to the same constraints as P recovery:
- it can only realistically be recovered at relatively high concentrations, e.g. from the sludge return streams if implemented at a sewage treatment works, and
- the recovered product is primarily used in agriculture, and has a financial value.
But there, the similarities end. In the sludge return streams, P arises mainly as phosphate, which forms insoluble salts. Ammonia can be removed along with P as struvite, a useful fertiliser product for use in agriculture, but this generally takes care of only a small proportion of the ammonia (15% or less, depending on the P concentration). It’s possible to bump this up a little by chemical dosing, but this isn’t generally economically viable.
Ammonia is highly soluble in neutral solutions, forming the ammonium ion (NH4+). Although oxidising it away biochemically (nitrification) or chemically (so-called ‘breakpoint’ chlorination) is relatively straight-forward and reasonably cost-effective, extracting it for reuse is more challenging. The principal options appear to be:
- adsorption – using a selective ion exchange resin to remove the ammonium ion (Zhang & Liu, 2021)
- stripping – using either aeration or steam to desorb molecular ammonia (NH3) (Powders et al, 2025)
- acid extraction – using hydrophobic membranes to extract molecular ammonia into an acid solution (Li et al, 2024)
- electrically-assisted extraction – from applying an electric field and using selective bipolar membranes (Lee et al, 2022), and
- capacitive deionisation – using a combination of an electric field and porous electrodes for selective electrically-assisted adsorption and desorption (Deng et al, 2024).
The challenge imposed by all of these processes is that they all, to a greater or lesser extent, incur some sort of environmental penalty and cost. Adsorption demands chemicals for regenerating the resins and recovering the ammonia, which then has to be extracted from the regenerant. Stripping requires energy for aeration or steam generation. Electrically-assisted extraction and capacitive deionisation both obviously demand electrical energy, along with some rather specialist materials. And most of these processes require pH adjustment at some stage to shift the equilibrium between ionised (NH4+) and molecular (NH3) ammonia. And, of course, they’re not without a significant cost – particularly for the advanced electrically-driven separation processes.
But that’s not all. As with phosphorus – or, indeed, just about any other prospective resource in wastewater – ammonia can most effectively be recovered at relatively high concentrations. This is purely down to equilibrium thermodynamics: the more of the stuff there is in the water, the more that can be proportionally removed.
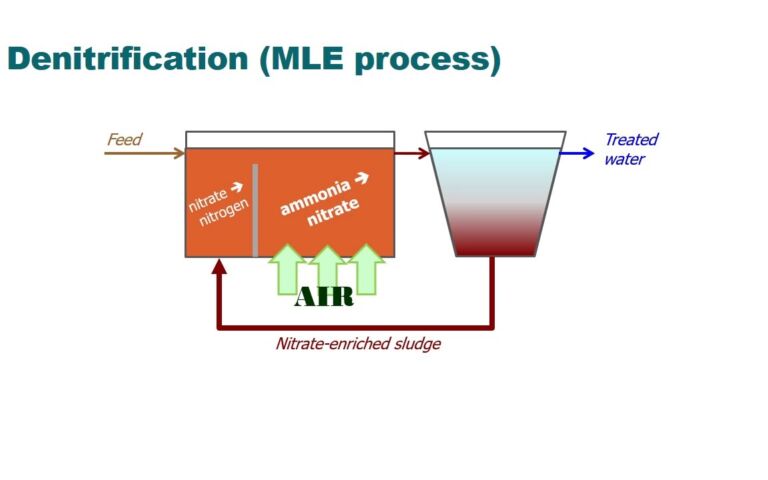
The ammonia concentration in sewage is normally below 50 mg/L, although it’s much higher in some industrial effluents like livestock manure wastewater and landfill leachate. In a municipal wastewater treatment plant, the highest ammonia concentrations are associated with the anaerobic digestion (AD) sludge liquor return streams. The AD itself is routinely fed with sludge from the primary and secondary stages (the waste activated sludge or WAS), the secondary stage being the biological process where nitrification – and, most often, denitrification by the Modified Ludzack−Ettinger process – is carried out. So, to be able to recover ammonia from streams where its concentration is high, it’s necessary to generate the WAS using the same aeration-intensive nitrification process that ammonia recovery is intended to avoid.
Having said this, ammonia recovery has been, or is in the process of being, piloted or implemented at full-scale at a few wastewater treatment and municipal solid waste management sites around the world. Thermal ammonia removal directly from high-strength wastewaters is well established: there are around 15 such plants, supplied by the UK company Organics, for landfill leachate treatment in Hong Kong alone. However, it is unclear how extensively ammonia recovery has been implemented. And while a few high-profile projects have been cited (Table 1), none of them appear to refer to full-scale implementation at a municipal wastewater treatment works.
| Location | Plant name | Technology | Product | Application |
|---|---|---|---|---|
[a] Demonstration scale, MSW Municipal solid waste | ||||
| Copenhagen, Denmark | Amager Bakke (Copenhill), MSW | Thermal ammonia stripping + acid scrubbing | Ammonium sulphate | Agricultural fertiliser |
| Hamburg, Germany | Hamburg WwTP (REPHOS)[a] | Air stripping + acid absorption | Ammonium sulphate | Agricultural fertiliser |
| Osaka, Japan | Osaka City WwTP | Ammonia stripping and recovery | Ammonium salts (varied forms) | Agriculture & industrial processes |
| Tyneside, UK | Howdon WwTP[a], sludge return liquors | Thermal ammonia stripping (Organics) | Ammonium solution | Fertiliser, fuels |
Which means that the demonstration plant (Fig. 1) at the Howdon site in the UK could be the first of its kind globally, since it specifically recovers ammonia (as a 20% solution) from municipal wastewater sludge liquors (0.3% ammonia). This can all be achieved with low-grade heat from the site: no pH adjustment required. And none of those pesky membranes either.
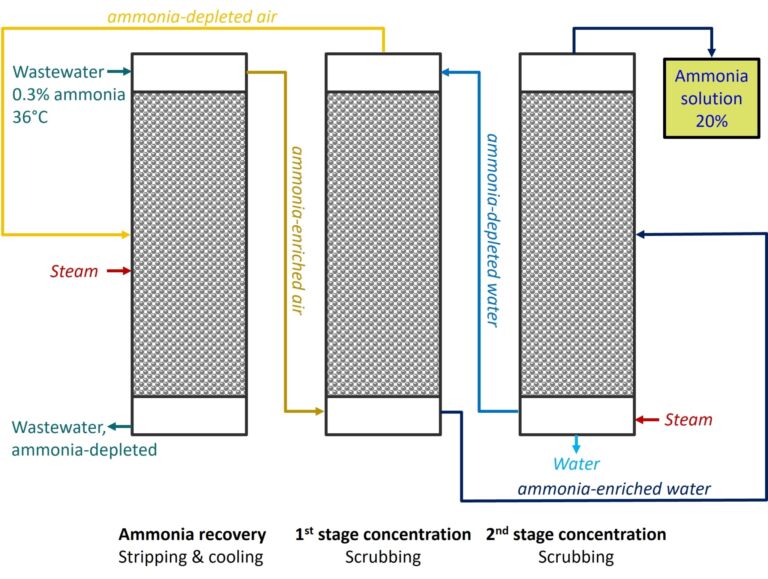
Ammonia is not a limited resource, unlike phosphorus. However, its synthesis (by the Haber process) is energy-intensive, making its production from renewable sources attractive – if somewhat expensive (currently about double the price of conventionally-produced ammonia). It’s also extremely versatile in its applications, which include fertilisers, the synthesis of industrial chemicals (nitric acid and hydrazine, to name but two), cleaning products, refrigeration, pharmaceuticals production and even as a fuel. Given the potential efficiency gains in ammonia production from renewable sources, including the use of membranes for enhanced mass transfer in the contacting towers, it seems only a matter of time before there is parity in the supply cost between renewably and non-renewably generated ammonia.