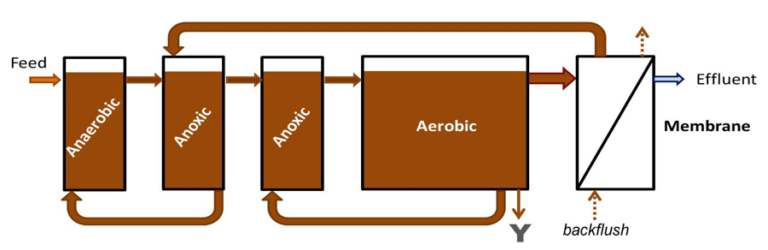
MBR operation and maintenance − nutrient removal

N removal
Nitrate formed from nitrification of ammonia can be converted to nitrogen gas by biochemical reaction with organic carbon, a process known as ‘denitrification’. This can only take place if dissolved oxygen is absent but nitrate is present − conditions described as ‘anoxic’.
Returning the treated nitrate-rich sludge to an anoxic zone in front of the aerobic treatment step allows the nitrate to be removed, a process modification known as the 'Modified Ludzack−Ettinger' (MLE) process. A combination of aerobic nitrification and anoxic denitrification permits most of the total nitrogen (TN) to be removed.
MBR operation and maintenance − nitrogen removal
P removal
If there is no source of oxygen to allow any aerobic or anaerobic biochemical reaction with the organic carbon to take place − either from oxygen or oxyanions − then the conditions are described as ‘anaerobic’.
In the treatment of municipal wastewater, anaerobic conditions are applied as a treatment step in assisting the removal of phosphate from the water. Biological phosphorus removal (BPR) in this manner proceeds through recirculation of the sludge to an anaerobic zone. This promotes phosphate removal in two ways:
- by encouraging the phosphate to be taken up by the biomass (the microbiologically active component of the mixed liquor), and
- by releasing the biological phosphate as inorganic phosphate, which can then be removed by chemical precipitation.
So biological phosphorus (P) removal exerts further energy demand for sludge transfer to the anaerobic tanks, as well as chemical demand for the precipitation.