Waste products? The circular economy in wastewater treatment


Simon Judd has over 35 years’ post-doctorate experience in all aspects of water and wastewater treatment technology, both in academic and industrial R&D. He has (co-)authored six book titles and over 200 peer-reviewed publications in water and wastewater treatment.
The idea of waste becoming a resource is hardly a new one. Recycling of glass containers, aluminium cans and (to a more limited extent) plastic drinks bottles has been around for decades, and remains justifiably a high priority. Composting of garden waste has been around for centuries.
But what of wastewater? Once again, recovery of resources from wastewater is hardly a new topic. Reclamation of water for non-potable use (and, in some parts of the world, potable water supply) is very well established. This is one of the key applications of MBRs, often upstream of UV irradiation, activated carbon adsorption and/or reverse osmosis. We’ve been using anaerobic digestion to convert organic carbon in sewage sludge sources to methane for decades, the latent energy often being recovered directly in combined heat and power (CHP) engines. The sludge itself, once pasteurised or otherwise rendered inoffensive, can be used as a fertilizer − though this is by no means universally accepted as an end disposal route.
So, what else is there? Well, sewage is, in principle, rich in resources: there's a lot of different stuff that ends up getting flushed down the toilet or washed into the sewers and drains. As well as the organic carbon, there are plenty of potentially useful inorganic compounds.
First up are the nutrients: nitrogen (N) and phosphorus (P). P recovery has been extensively explored, which seems sensible enough given the extremely tight P discharge limits imposed in many parts of the world and, by implication, the potentially prodigious amounts of P in the collected sludge solids from wastewater treatment.
Nitrogen (N) recovery is a bit more tricky. Whereas P forms an insoluble solid (like struvite or aluminium phosphate) that can be dropped out of solution, N does not. Ammonia in wastewater is classically removed by nitrification and denitrification − usually by the Modified Ludzack Ettinger (MLE) process. Inorganic ammonia is actually ionic in neutral solutions, so can in principle be removed by ion exchange. This is reasonably efficient provided an adsorption media of sufficiently high capacity can be identified. And as it happens, certain zeolites will do the job quite nicely, and can be regenerated on site using brine. The brine then has to be stripped of the ammonia (apparently achievable using yet another membrane process: there's a lot of membrane technology in water reclamation).
Then there’s the metals: it’s been mooted that there’s a sufficient precious metals content in municipal sludge to make it worthwhile extracting them. And for some industrial operations, the recovery of the high-value residual materials from the wastewater is sufficient to justify implementing the advanced wastewater treatment technology. This would include things like copper, zinc and nickel from metal plating wastewater. There are even moves towards implementing coagulant recovery from potable water sludge.
But it appears that it’s possible to go one step further. Not all of the organic matter can be converted to biogas. The residual organic solids, essentially the non-biodegradable component of bathroom tissue and cotton-based textiles, can be recovered as a lignocellulosic polyhydroxyalkanote (PHA) bioplastic product. This isn't something which can be directly recycled as bathroom tissue but is nonetheless tangibly reusable, for things like building materials.
As is always the case with these fine ideas, so much depends on the bottom line. Reclamation is usually seen as a good thing, particularly if the net energy requirement is lower than the conventional approach. Clearly phosphorus recovery must be at least close to being economically competitive: it’s already being implemented at full scale at some sites. But what about the others?
Looking at ammonia recovery and reuse, the alternatives both consume significant amounts of energy. Ammonia in the wastewater is classically converted to nitrate by aeration, at a nitrogen:oxygen mass ratio of more than 4.5. Some of this energy can then be recovered by denitrification, which ultimately generates nitrogen. So a lot of energy (over 2 kWh per kg N) is used just to convert something potentially useful (ammonia) to something innocuous (nitrogen gas) and of no value whatsoever. Ammonia itself is produced by the Haber-Bosch process, which consumes anything between 9 and 13 kWh per kg N. Summing these figures clocks up a staggering 11−15 kWh per kg N. It would seem inevitable that recovery of ammonia from wastewater would come in a lot less expensive than this.
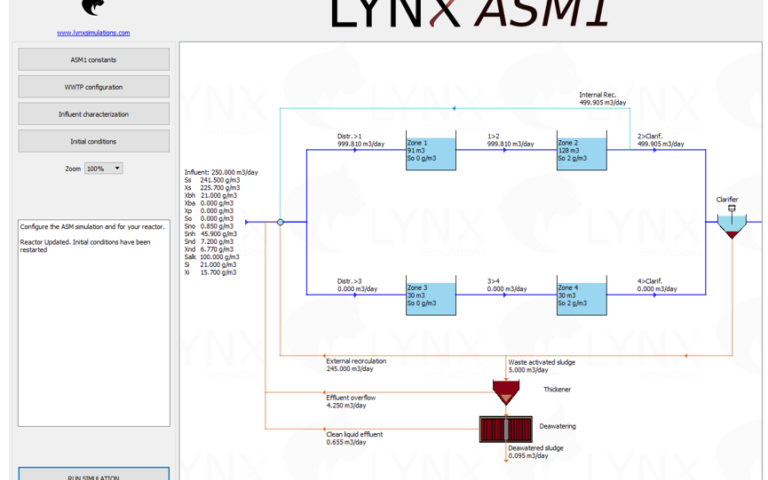
And there's yet more good news. Anaerobic treatment of sewage (a) generates methane as the main carbon product instead of CO2, and (b) leaves the ammonia unconverted, ready to be recovered and reused by zeolite-based ion exchange (Sancho et al, 2017). It would seem a no-brainer: cease this wasteful aerobic treatment immediately and switch to anaerobic treatment to recover the energy, N, P, water (Fig. 1) and maybe that PHA bioplastic for good measure.
If only it were that simple ...
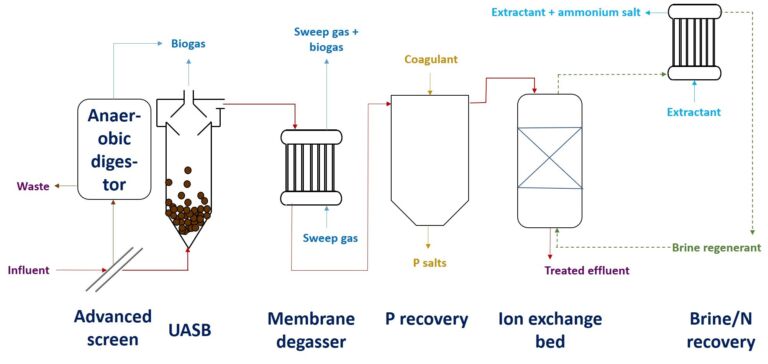
It’s actually specious to consider energy consumption normalised against the amount of material recovered, because the quantities of nutrient are small. The true figure is the energy demand per unit volume or mass of wastewater treated. Even for notionally low-energy anaerobic treatment, energy is still demanded for pumping, and there are other cost components too.
Indeed, the challenges of anaerobic treatment of sewage have been discussed in these pages before, and not a lot has changed in the intervening three years. Anaerobic processes are still slower than aerobic ones, demanding larger tanks. The tanks must be sealed to exclude oxygen, increasing installation costs. The methane generated is soluble in water, and so must be stripped − demanding another potentially costly downstream process. And, yes, membranes can be used for that too (Cookney et al, 2016). As for the ammonia reclamation, the value of the small amounts of reagent recovered is almost inconsequential compared to the cost of the zeolite media bed and the associated equipment required to recover the ammonia from the brine.
However, it doesn't do to be too picky. The sad fact is that the need for low-carbon alternatives to all industrial operations, including wastewater treatment, is becoming critical. Whilst process flowsheets such as the one above may seem fanciful and unattractively priced, it is an example of the sort of disruptive technology options that need to be seriously considered to move the world away from the high-carbon operations that have been the modus operandi for decades and are ultimately the root cause of global warming. The only real impediment is complacency.