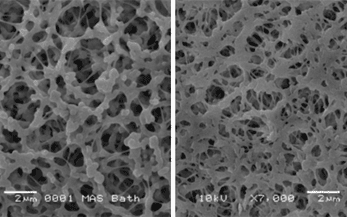
Industrial MBRs − pharmaceutical sector

Membrane bioreactors in the pharmaceutical sector
Concerns over the occurrence and detrimental impacts of active pharmaceutical ingredients (APIs) in the environment as a whole were originally raised in the late 1990s, although these compounds have been detected in rivers since the mid-1990s and more recently in seawater. The biorefractory nature of many pharmaceutical compounds has meant that they tend to accumulate in the environment, albeit at very low concentrations, and it is their persistence and potential for arising in potable waters which has caused widespread concern.
A large proportion of the active pharmaceutical ingredient (API) load in effluents generally derives from municipal wastewaters. A very large number of publications have appeared in learned journals on the fate and concentration of APIs in municipal wastewater and wastewater treatment plants, and CAS plants in particular. Less attention has been focused on the impact of pharmaceutical effluents. However, effluents from pharmaceutical production are regulated and subject to limits on discharged APIs; the primary challenge to effluent treatment arises from the ancillary constituents, such as the organic solvents, fermentation broth solids and additives, which are higher in concentration and can be more recalcitrant.
Pharmaceutical activities and wastewaters
Within the pharmaceutical industry, a number of different activities exist whose generated effluents vary widely. Fermentation processes (i.e. through anaerobic biological treatment) and synthetic organic chemistry are both used to produce medicines and fine chemicals. These two processes are often combined in a single manufacturing facility. Biological production (i.e. the use of live animals) is used to create vaccines and antitoxins. Finally, there are plants or operations dedicated to the production of tablets, capsules and/or solutions, though mixing, formulation and preparation.
As with many other industrial sectors (such as the food and textile industries), production varies regionally and seasonally according to demand. Wastewaters generated also vary significantly in quality according to the type of plant, with fermentation and organic chemical synthesis wastewaters generally being the highest in organic load and the most challenging to treat. Drug formulation, on the other hand, tends to produce more readily treatable wastewaters.
Treating pharmaceutical wastewater
Treatment of pharmaceutical wastewater draws parallels with both food industry wastewaters, in particular fermentation effluents, and landfill leachate. This is because fermentation is used for pharmaceuticals production and landfill leachate similarly contains high levels of biorefractory organic compounds.
In general, the biological treatability of pharmaceutical wastewaters decreases with decreasing BOD/COD ratio, API concentration, and the concentration of the raw materials, intermediates and solvents.
Moreover, above a COD concentration of ~4,000 mg/L CAS may be considered unsuitable (Suman Raj and Anjaneyulu, 2005). Otherwise, aerobic treatment is made viable through employing long HRTs and SRTs, which are made more accessible by the use of MBRs. HRTs reported for MBR trials conducted at bench- and pilot-scale on analogue and actual pharmaceutical wastewaters varied from 1 to >6 days, with SRTs of 26−100 days. In all cases, reported removal of COD exceeded that of the target pharmaceutical(s), with COD removal normally exceeding 90%.