Removal of Emerging Trace Organic Contaminants (TrOCs) by MBR

Faisal I. Hai
Faisal Hai works at the Strategic Water Infrastructure Laboratory, School of Civil, Mining and Environmental Engineering, The University of Wollongong, Australia. He is also co-editor of ‘Membrane Biological Reactors: Theory, Modeling, Design, Management and Applications to Wastewater Reuse’. Contact Faisal at faisal@uow.edu.au.
1. Introduction
Trace organic contaminants (TrOCs) in municipal wastewater consist of a wide range of naturally occurring and synthetic chemicals (Luo et al, 2014). They include industrial chemicals, chemicals used in households, chemicals excreted by people, and chemicals formed during wastewater and drinking-water treatment processes. Some key classes of TrOC include pharmaceuticals and personal care products (PPCPs), natural and synthetic hormones, perfluorochemicals and nanoparticles, pesticides or their metabolites and synthetic industrial organic chemicals.
TrOCs are biologically active and can thus present a threat to the aquatic environment with effects such as acute and chronic toxicity to aquatic organisms, accumulation in the ecosystem and loss of habitats and biodiversity, as well as a range of possible adverse effects on human health. Modern sewage treatment plants can effectively remove total organic carbon (TOC) and total nitrogen (TN) as well as achieve some degree of disinfection. However, these plants have not been specifically designed to remove TrOCs (Hai and Yamamoto, 2011; Judd, 2011).
This article casts light on the factors, mechanisms and extent of TrOC removal by membrane bioreactors. In particular, it compares the performance of MBRs with other conventional biological processes, and provides a brief overview of MBR-based combined technologies for efficient removal of TrOCs.
2. Relative performance of MBR and other biological processes
2.1 Conceptual expectations
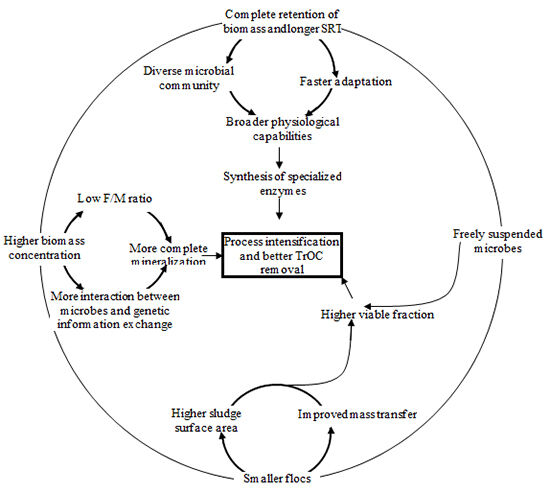
MBRs can potentially achieve better removal of TrOCs than conventional activated sludge (CAS) processes due to their ability to operate under longer solids retention times (SRT), higher biomass concentrations and complete retention of suspended solids. Compared to CAS processes, MBRs may provide additional removal mechanisms for TrOCs:
- the membrane serves as an effective barrier to solids, onto which many TrOCs can adsorb, and this cake layer on the membrane can potentially prevent the escape of some extracellular enzymes and soluble oxidants creating a more active biological mixture capable of degrading a wider range of carbon sources, and
- the longer SRT in MBR may facilitate additional biological transformation of TrOCs (Figure 1).
2.2 Reported comparative performance of CAS and MBR
A number of investigations have been conducted comparing the removal efficiency of TrOC from wastewater by the CAS processes and advanced MBR treatment. These two treatment options demonstrate compound-specific comparative performance. Occasionally TrOC removal efficiency has been observed to be very similar, and high for both treatments (e.g. for ibuprofen, naproxen, acetaminophen and paroxetine), while some compounds such as the anti-epileptic drug carbamazepine and diuretic hydrochlorothiazide can pass through both the systems untransformed (Cirja et al, 2008, Oulton et al, 2010).
However, some studies point to improved removal efficiency by MBR compared to CAS treatment. While MBRs may not necessarily always yield higher removal efficiencies, they are nevertheless more advantageous because they exhibit a more consistent performance and shorter lag times, indicating a superior response to fluctuating influent concentrations. Additionally, MBRs offer the advantages of increased plant flexibility through more compact installations and allow modification of design to fine-tune biological performance.
3. Factors affecting TrOC removal by MBR
3.1 TrOC characteristics
TrOCs can be removed from aqueous solution by four broad mechanisms, namely:
- volatization
- sorption
- photolysis, and
- biodegradation.
These mechanisms are governed by the physicochemical properties of the TrOC and the operating condition of the MBR system. In general, because of the very high suspended solid content in the reactor and the limited exposure to sunlight (ultraviolet radiation) during MBR treatment, the contribution of photolysis to the overall removal of TrOC during MBR treatment is negligible. TrOC can be transferred from the aqueous phase to the gas phase, if they are sufficiently volatile, or to the solid phase, if they are sufficiently hydrophobic. Volatization may become significant when the Henry’s law constant of the compound is more than 10-3 (atm.m3/mol), particularly under aeration conditions.
The hydrophobicity of an organic compound can be estimated by log D or the effective octanol-water partitioning coefficient. In general, sorption to biosolids has been observed as a major removal mechanism when log D value of the TrOC is about 3 or higher. In a specific system, where the solid phase is well defined, solid water partitioning coefficient (Kd) is commonly used to experimentally describe the sorption of the TrOC onto solids. It has been suggested that for compounds showing a log Kd below 2.5, sorption onto biosolids is not relevant and their removal will consequently be governed by biodegradation (Joss et al, 2005).
A coupling effect between biosorption and biodegradation is possible because higher biosorption means longer time for biodegradation to occur. Suarez et al (2010) proposed that compounds could be classified according to their kbiol into:
- very highly (kbiol > 5 L/(g SS. d))
- highly (1 < kbiol < 5 L/(g SS. d))
- moderately (0.5 < kbiol < 1 L/(g SS. d)) and
- hardly (kbiol < 0.5 L/(g SS. d))
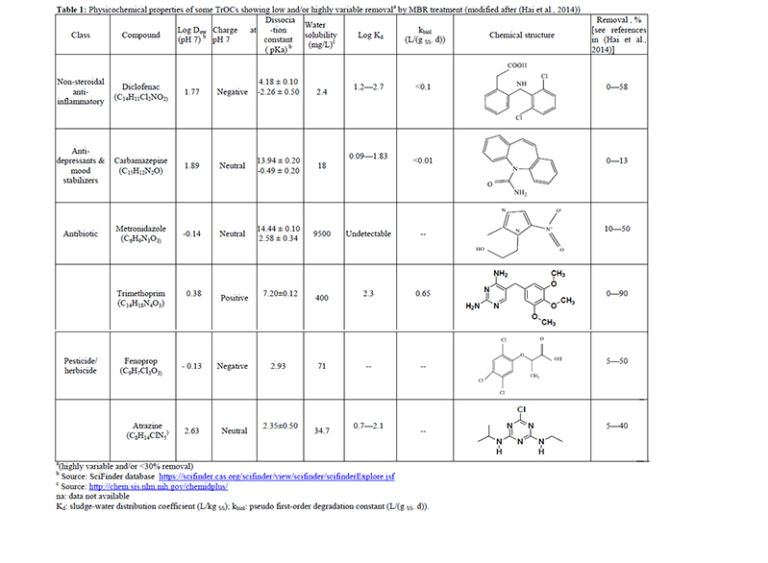
biodegradable. Recent research has successfully demonstrated the connection between the molecular structure and/or functional moieties of TrOC and their biodegradability during MBR treatment (Tadkaew et al, 2011). Tadkaew et al (2011) systematically studied the influence of electron withdrawing groups (EWGs) and electron donating groups (EDGs) on TrOC removal and proposed a qualitative framework for prediction of TrOC removal by MBR. In their study. high removal efficiencies were observed with most compounds bearing EDG such as hydroxyl and primary amine groups. It is noted that the poor removal by MBR treatment of the TrOC listed in Table 1 appears consistent with their low hydrophobicity (log D or log Kd) and/or low biodegradability (kbiol).
3.2 Operating parameters
Solids retention time (SRT)
The improvement of biodegradation of various TrOC with the increase in SRT can be explained by two possible mechanisms:
- the microbial community may become more diversified with increasing SRT,
- the microorganisms may diversify their metabolic activity in response to the lower sludge loading with bulk organics.
The minimum SRT required for high removal may vary between 5 d (e.g., caffeine and oxybenzone) to 15 d (e.g., galaxolide and tris(2- chloroethyl)phosphate) depending on the TrOC. As noted earlier, biological transformation of TrOC is not only a function of their kbiol values, but also of their Kd values, since biodegradable compounds with large Kd values (log Kd >2.5) will be removed when the SRT in the aeration tank is long enough to accomplish their biodegradation. However, once a certain limit value of SRT is exceeded, the removal efficiencies are not enhanced anymore (Suarez et al, 2010) (Table 2).
Mixed liquor pH and temperature
Wastewater pH can influence TrOC removal by influencing both the physiology of the microorganisms (optimum pH for enzymatic activity) and the solubility of ionisable TrOCs in wastewater. Ionisable TrOCs can exist in different protonation states at different pH, which can lead to different extents of hydrophobicity (log D) at different pH values (Tadkaew et al, 2010). Despite the possibility of enhanced adsorption of certain ionisable TrOC on sludge, acidic pH may have adverse impact on certain microbial groups, which may in turn lead to reduction of TOC, TN and/or total phosphorous (TP) removal.
Table 1: Physicochemical properties of some TrOCs showing low and/or highly variable removal by MBR treatment (modified after (Hai et al, 2014)) (larger size PDF available).
| Kd | kbiol | Dependence on SRT | Removal |
|---|---|---|---|
| High | High | No | High |
| High | Low | Yes | Moderate to high |
| Medium | High | Yes/No | Moderate |
| Low | Low | No | Low |
Because microbial growth and activity as well as solubility and other physicochemical properties of organics are significantly affected by temperature conditions, temperature variability has been related to deterioration in bulk water quality parameters and system instability. Through experiments conducted in a lab-scale MBR, Hai et al (2011) provided unique insight into the effect of dynamic short term (diurnal) temperature variation on TrOC removal by MBR treatment. With a few exceptions, operation at 45°C clearly exerted detrimental effects on the removal efficiency of the TrOC selected in that study. The removal of most hydrophobic compounds (log D > 3.2) was stable during operation under a temperature range of 10−35°C. On the other hand, for the less hydrophobic compounds (log D < 3.2) a comparatively more pronounced variation between removals in the lower temperature regimes (10−35°C) was observed. Lower and more variable removal efficiency was seen for certain hydrophilic compounds at 10°C which have been reported to be moderately recalcitrant to MBR treatment.
Mixed liquor dissolved oxygen (DO) concentration
Mixed liquor DO concentration (‘redox conditions’) affect biodegradation. Degradation can occur under aerobic (molecular oxygen available), denitrifying (no molecular oxygen available, nitrate available), or anaerobic (neither molecular oxygen nor nitrate available) conditions. Different TrOC removal efficiencies have been observed for anaerobic, anoxic and aerobic conditions. Low oxidation reduction potential (ORP) or DO (i.e., anoxic/anaerobic) regimes are conducive to biodegradation of some TrOCs. However, often an important prerequisite to anoxic biodegradaton of TrOCs is internal recirculation between the anoxic and aerobic bioreactors, in the absence of which anoxic/anaerobic regimes alone may only enhance biosorption. Nevertheless, a major role of the aerobic regime has been consistently observed (Phan et al, 2014). Particularly, there is circumstantial evidence linking nitrification to a unique capability to biodegrade TrOCs.
4. Post treatments and MBR-based hybrid systems
The low removal efficiency of biologically persistent and hydrophilic TrOC necessitates the integration of MBR with several other post-treatment processes to ensure adequate removal of TrOC.
4.1 Combination with physicochemical processes
Because MBRs can produce effluent with much lower bulk organic content when compared to CAS, significant synergy can be realised when it is integrated with other advanced treatment processes (Alexander et al, 2012). In addition, given the small physical footprint of the MBR process, it is possible to deploy these integrated systems for decentralised water recycling applications. This section provides a brief overview of the integration of advanced treatment processes including activated carbon adsorption, NF/RO, UV oxidation, and ozonation with MBR treatment for TrOC removal. Schematic diagrams of these MBR-based hybrid systems are shown in Figure 2.
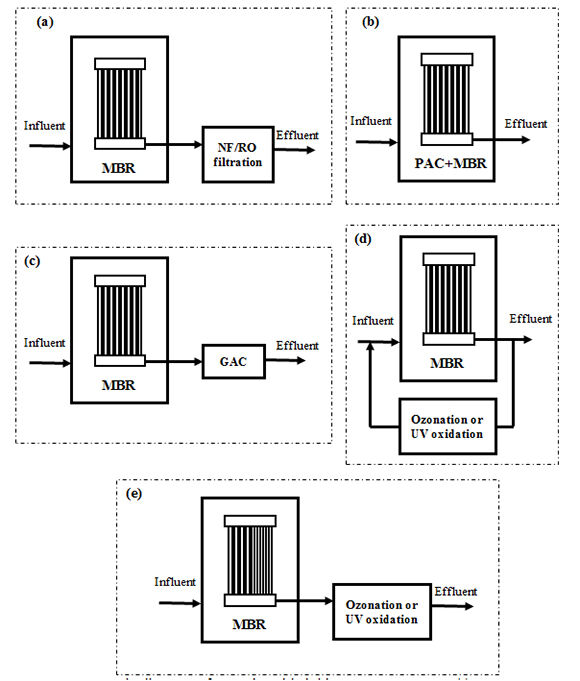
The advantages of combining MBR and NF/RO treatment with respect to the removal of TrOC have been demonstrated by a few recent studies (e.g. Alturki et al, 2010). Apart from post treatment of MBR effluent, recent developments integrating NF (Zaviska et al, 2013) or forward osmosis (Alturki et al, 2012) membranes with bioreactors are also worth mentioning.
Activated carbon adsorption can be used in conjunction with an MBR system in two different configurations:
- addition of powdered activated carbon (PAC) directly into the reactor of the MBR (Li et al, 2011, Nguyen et al, 2013a), and
- post-treatment of the MBR permeate using either a granular activated carbon (GAC) column (Nguyen et al, 2013b; Nguyen et al, 2012) or a continuously mixed reactor containing a slurry of PAC.
Nguyen et al (2013a) conducted a preliminary comparison between the PAC−MBR and MBR−GAC configurations and reported that the former can perform better in terms of activated carbon usage.
Similar to activated carbon adsorption, due to low SS and background TOC concentration in MBR permeate, an advanced oxidation process (AOP) can be efficiently coupled with MBR treatment. Two reactor configurations can be perceived for such combinations:
- side stream treatment with internal recirculation between MBR and AOP, and
- post-treatment of the MBR permeate.
Although any oxidation treatment process can be integrated with MBR treatment in this fashion, most studies available to date have been concerned with ozonation and UV (or UV in combination with TiO2 or H2O2). Biodegradation and oxidation can complement each other for the removal of a specific TrOC (Laera et al, 2012, Nguyen et al, 2013c). However, this approach would lead to an increase in both the investment and operational costs. It is also noteworthy that most of the data available to date are from laboratory scale studies. Further research is still needed for full scale validation, scaling up, system optimization, and to improve our overall understanding of the complementarities between MBR and AOPs.
5. Bioaugmented MBR for TrOC removal
Well-acclimated microbial strains capable of either using TrOC as the sole source of carbon or degrading them by cometabolism can be cultivated and later introduced into large-scale biological reactors, hence implementing bioaugmentation for TrOC removal. Despite the great potential of such an approach, only a few such studies could be identified in the literature. Liu et al (2008) reported efficient (above 90%) and stable atrazine removal for around two months in an MBR bioaugmented with genetically engineered Escherichia coli containing an atrazine chlorohydrolase gene. Ghyoot et al (2000) examined the behaviour of a 3-chlorobenzoate (3CBA) degrading Pseudomonas putida (BN210) in a CAS and an MBR system. The MBR showed higher resistance towards shock loading of 3CBA in terms of improved COD removal. Notably, based on recent studies confirming significant removal of various TrOC by pure ‘white-rot’ fungal cultures (Yang et al, 2013b), there have been a few attempts of development of an MBR containing a mixed microbial community including the white-rot fungi (Nguyen et al, 2013d, Yang et al, 2013a).
6. Conclusion and future outlook
This article highlights the potential advantages of the MBR process over conventional CAS ones for the biodegradation and treatment of TrOC. Performance of MBR over CAS depends greatly on the conditions and the particular TrOC being investigated, and reports in the literature vary.
The situation is often complicated not only by operational parameters but also by the vastly different reported removals for a single TrOC. Reasons for this can be many, including differences in initial TrOC concentrations, primary substrate concentrations, incubation times, and microbial inoculum sources.
There is a dearth of information on the extent of TrOC biodegradation and its pathways. Another area that merits further research is deciphering the relationships between microbial community and TrOC degradation. Furthermore, bioaugmentation of microbes of higher degradation capacity with the indigenous microbes remains a mostly unexplored topic. In addition to attempts to fine-tune biodegradation of TrOC, development of hybrid systems having MBR at the core will be indispensable to treat the wide varieties of TrOC from wastewater.